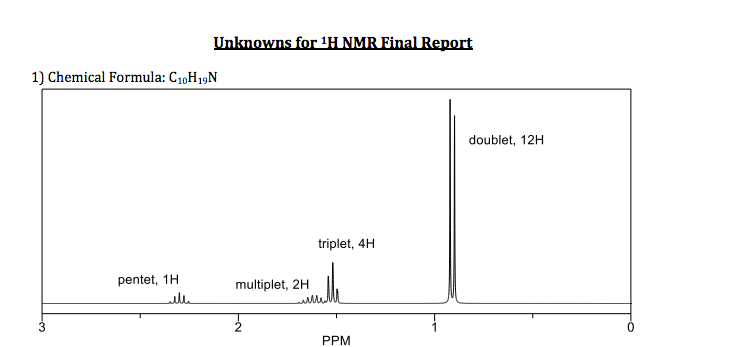
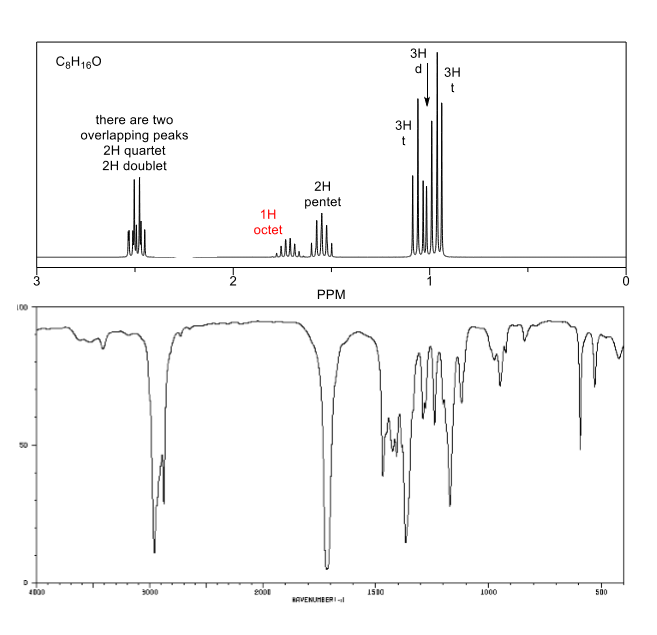
Pentet Nmr
Triplet of doublets description.
Pentet nmr. C nmr spectra or for signals in. H decoupled spectra that are coupledto other mag netically active nuclei. The splitting of signals in an nmr spectrum is due to nmr active bonded adjacent atoms. Idealized septet lines in a 1 6 15 20 15 6 1 ratio leaning septet.
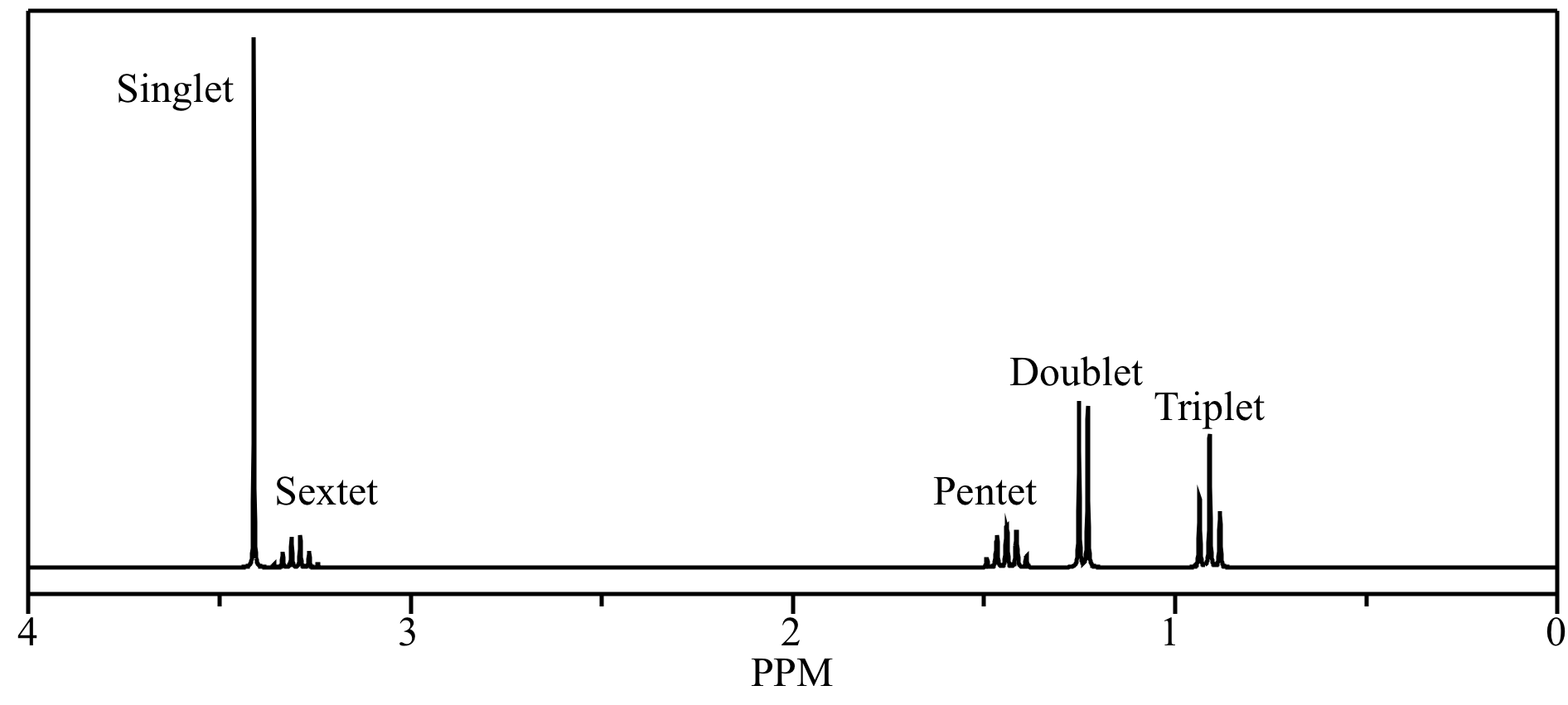
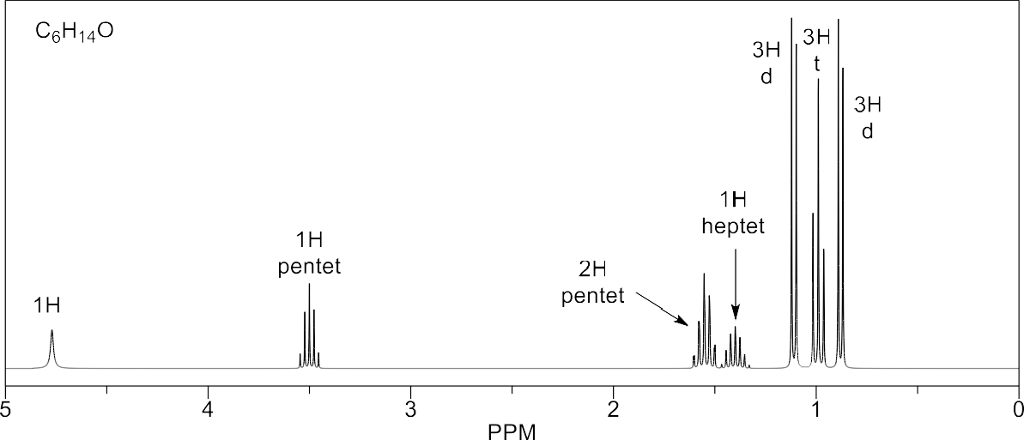
Click image for a larger version. For methanol solvent this corresponds to chd 2 od so a 1 2 3 2 1 pentet signal is observed at 3 31 ppm. This simulated 1 h nmr spectrum of sec butyl ether has a pentet at 1 44 ppm due to the molecule s ch 2 group. C nmr signal will be considered a singlet if the multiplicity is not assigned.
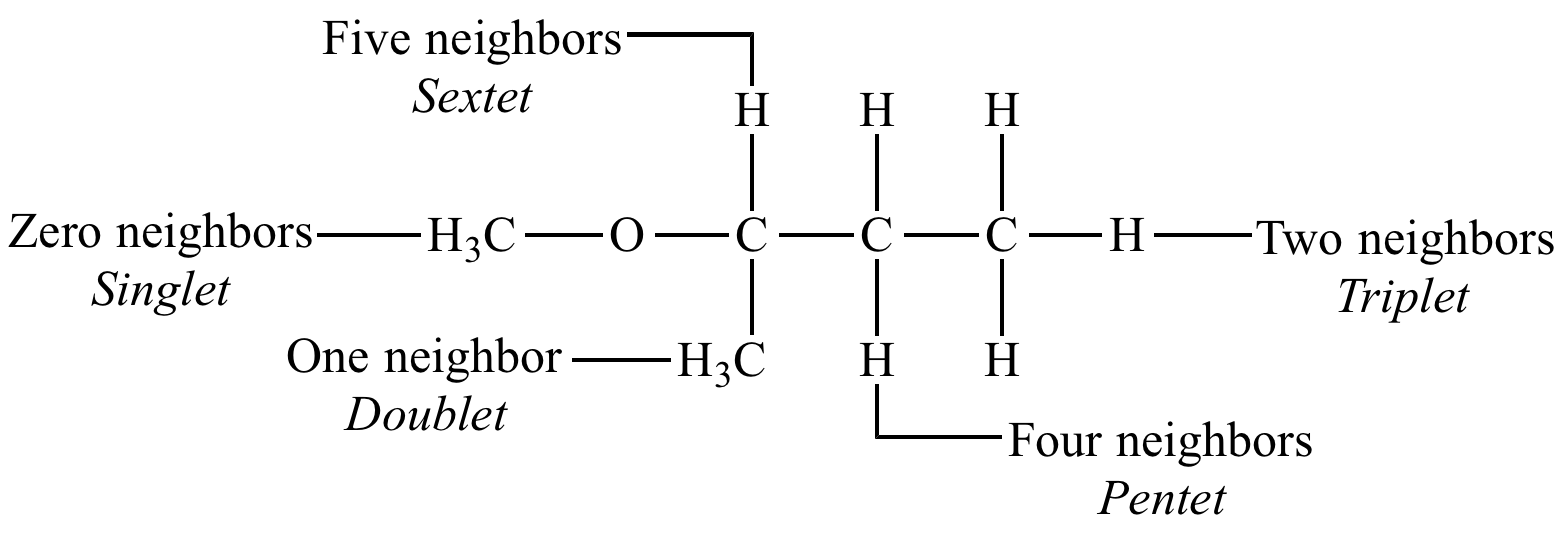
Recall that deuterium has a spin quantum number i of 1 so n deuterium atoms will split a proton signal into 2in 1 lines the same solvents are used for 13 c nmr spectra so the same rules about splitting patterns apply here also. 1 7 3 only rarely is a true multiplet observed in a. Click on the image for a larger version. The multiplicity of a signal in an nmr spectrum is given by the formula.
Only stereochemically different 1hs give different signals. The simulated 1 h nmr spectrum of 2 chloropropane has a septet at 3 68 ppm due to the methine proton. For instance both pentet and quintet are commonly used to describe a 1 4 6 4 1 splitting. Idealized pentet lines in a 1 4 6 4 1 ratio leaning pentet.
2i n 1 where i and n represent. Td j 10 3 hz the j value of the doublet is always the distance between the first and second. Further there are general inconsistencies in reporting formats within this journal with abbreviations such as s sept spt being used for septet. Proton nuclear magnetic resonance proton nmr hydrogen 1 nmr or 1 h nmr is the application of nuclear magnetic resonance in nmr spectroscopy with respect to hydrogen 1 nuclei within the molecules of a substance in order to determine the structure of its molecules.
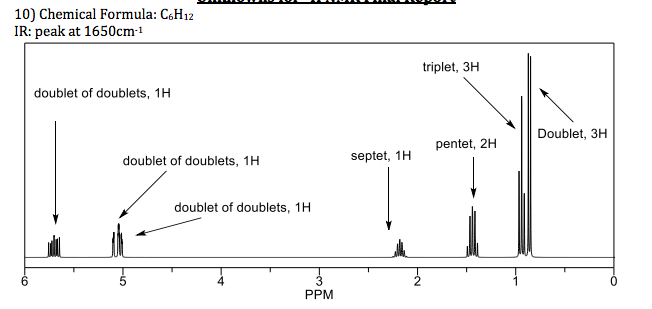
However a certain region may contain a group of unresolved. In samples where natural hydrogen h is used practically all the hydrogen consists of the isotope 1 h hydrogen 1. A triplet of doublets td is a pattern of three doublets in a 1 2 1 ratio of relative intensities that results from coupling to two protons or other spin 1 2 nuclei with a larger j value and one proton or other spin 1 2 nucleus with a smaller j value. Nuclear magnetic resonance spectroscopy nmr spectrum represents the different interactions of stereochemically different protons 1h with the applied magnetic field we will focus on 1h nmr proton h 4 general rules for 1h nmr spectra 1.